The Systems Biology of Human Diseases Laboratory is focused on both experimental and theoretical aspects of Cellular and Molecular Tissue Engineering, Metabolic Engineering, and Biomedicine with emphasis on clinical applications. Our research interests lie in the systems biology of metabolic diseases, specifically cancer. Our aim is to understand the role of metabolism in cancer progression, growth and metastasis. We use genetic and metabolic design principles to analyze healthy and diseased biological states. We are working to uncover the metabolic interactions between cancer cells and cells in neighboring tissue that support cancer growth and metastasis. Our research integrates both experimental and computational tools to develop a recipe for maintaining normal function of various organs. Our lab also makes a concerted effort to use engineering principles, such as multi-objective optimality and non-equilibrium thermodynamics to develop mathematical models for analyzing and understanding complex diseased states. The outcome of our work is to discover potential therapies that exploit metabolic vulnerabilities in cancer.
Developing Metabolic Therapies for Targeting Tumor Microenvironment
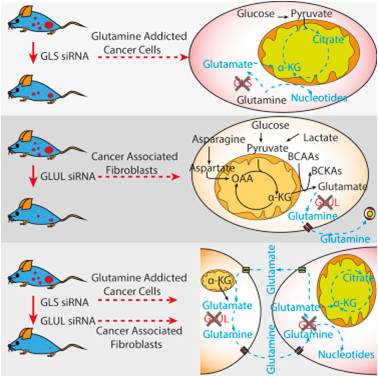
Stromal cells are an integral part of healthy tissue, and they are hijacked by cancerous cells to support tumor growth. They interact with cancer cells to help them grow and survive in harsh environments, resist drugs and invade surrounding tissue. Thus, targeting stromal cells becomes an important mode of therapy against cancer. Our lab has discovered that stromal cells supply essential metabolites to cancer cells by recycling metabolites secreted by cancer cells. Attacking this interaction can effectively eradicate tumors in mice! We have now taken on the challenge of identifying such metabolic interactions between stromal and cancer cells that can be targeted by drugs to improve therapeutic outcomes for cancer patients.
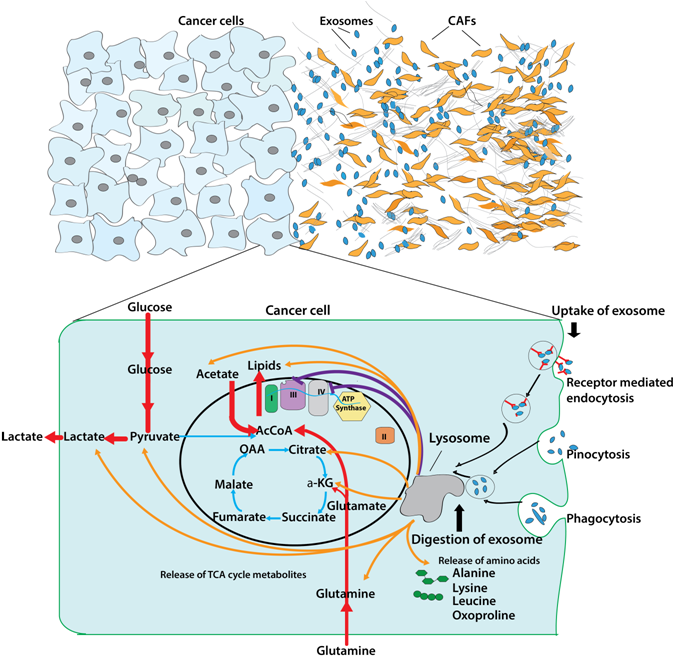
Dissecting Metabolic Influences of Tumor Microenvironment
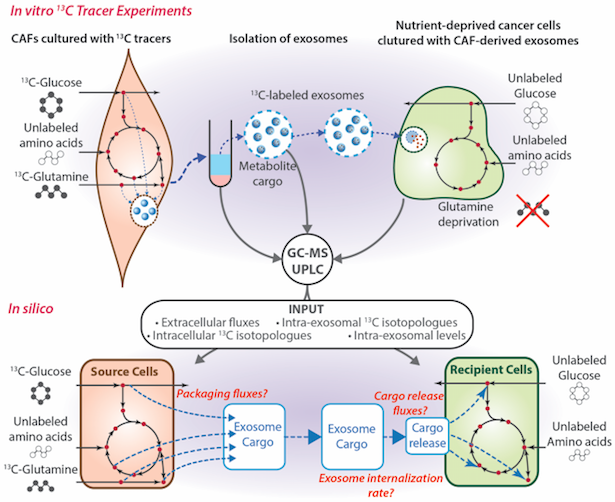
Non-malignant stromal cells are a major population of cells that make up the tumor microenvironment in solid cancers. They support the growth of cancer cells against all odds. One such supportive action is achieved via extracellular vesicles (i.e. exosomes) secreted by non-malignant cells that are internalized by cancer cells. Exosomes are vesicles that carry important biological material such as RNA, DNA, proteins and as our lab recently discovered – nutrients! These exosomes, therefore, act as care packages sent out by stromal cells to supply amino acids to nutrient-deprived cancer cells. We are now working to unravel how extracellular vesicles from different parts of the TME can support cancer cell metabolism, with a goal of targeting this important mode of communication
Investigating Cancer Metabolism using Computational Flux Analysis
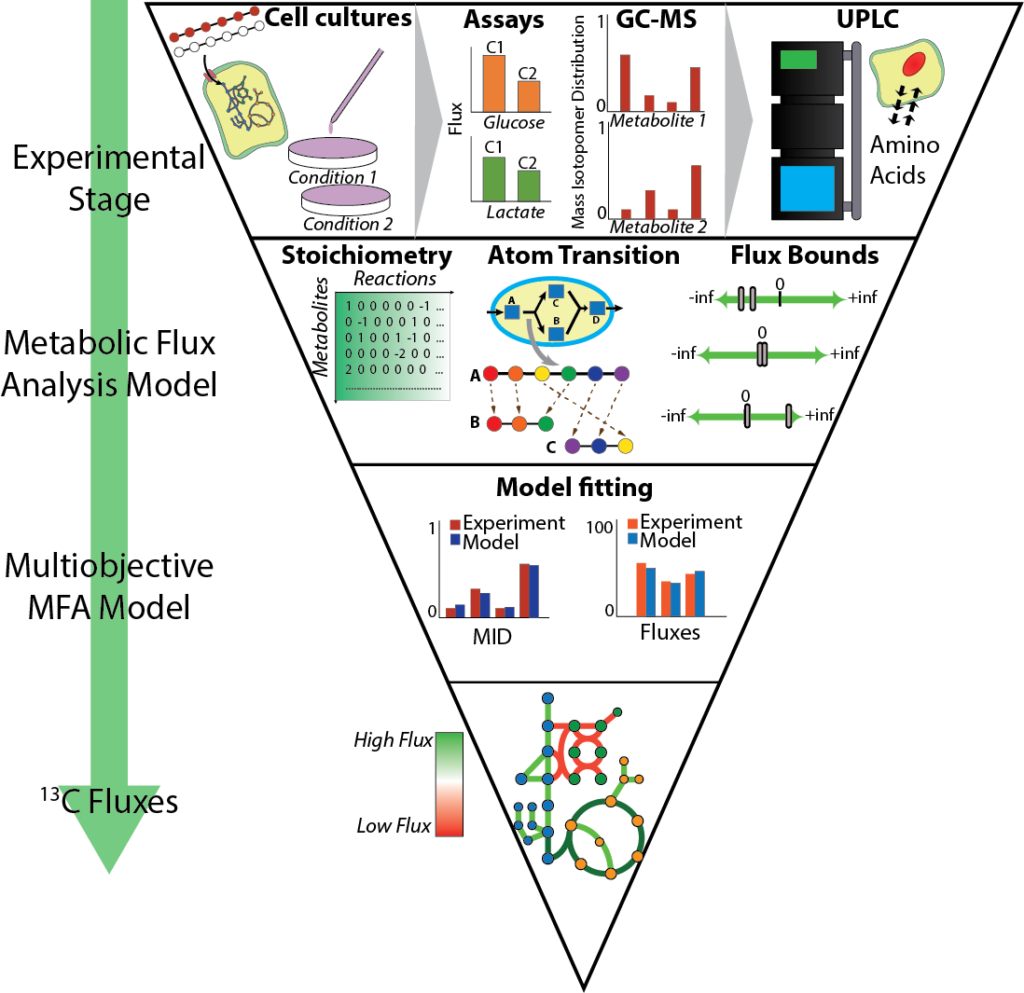
Cellular metabolism is a complex biochemical network that can be described by the physical and chemical laws that govern biochemical reactions. This provides us the opportunity to investigate cancer metabolism more deeply with the help of mathematical models driven by empirical data. By culturing cells in nutrients labeled with stable isotopes of carbon, nitrogen or hydrogen, we can follow their metabolic footprint inside cells! Computational algorithms such as 13-carbon metabolic flux analysis (13C-MFA) use metabolic footprints to recreate a quantitative picture of metabolic activity that experiments alone cannot provide. Our lab has implemented conventional 13C-MFA algorithms and developed novel MFA algorithms to obtain a quantitative understanding of cancer metabolism and pinpoint metabolic vulnerabilities.
Developing Novel Flux Analysis Tools to Dissect Metabolic Interaction within Tumors
13C-MFA tools have proven to be an indispensable tool to understand cellular metabolism. It has recently gained popularity among researchers focused on cancer metabolism. However, applying 13C-MFA tools built for bacterial and simple eukaryotic systems has proven challenging, especially since they are designed for population of same type of cells. Our lab is working on developing 13C-MFA-based algorithms with a focus on cancer metabolism and interactions among cancer and stromal cells. Exo-MFA and multicellular MFA will represent the next-generation of flux analysis tools that will go hand-in-hand with novel empirical techniques to understand and fight cancer.